Abstract
Background: Only about one third of AML patients with mutant IDH1 respond to IDH1 inhibitor monotherapy with a median response duration of 7 months, indicating the strong need for combination treatments. We have previously shown that BAY-1436032 is a highly effective oral pan-mutant IDH1 inhibitor, which has strong anti-leukemic activity in patient derived xenograft (PDX) models of IDH1 mutant leukemia in vivo. In the present study, we assessed the combination of azacitidine with IDH1 inhibitor BAY-1436032 in a preclinical PDX model of IDH1 mutated AML.
Methods: We tested the activity of BAY-1436032 alone or in combination with escalating doses of azacitidine in IDH1 mutated and IDH1 wildtype AML patient cells in vitro evaluating the effects on colony formation, apoptosis, cell cycle, differentiation and global gene expression changes. Leukemic cells from an AML patient with mutated IDH1, NPM1, FLT3-TKD and NRAS were xenografted in immunocompromised mice and treatment was started 28 days after transplantation. The control groups were treated with either vehicle, BAY-1436032 150 mg/kg once daily p.o. continuously, or azacitidine 1 mg/kg once daily s.c. days 1-5, repeated once after 28 days. The test groups were treated with BAY-1436032 and azacitidine in the doses mentioned above either starting both drugs on day 1 (parallel group) or starting azacitidine on day 1 but BAY-1436032 on day 6 (sequential group). The treatment was stopped after 84 days.
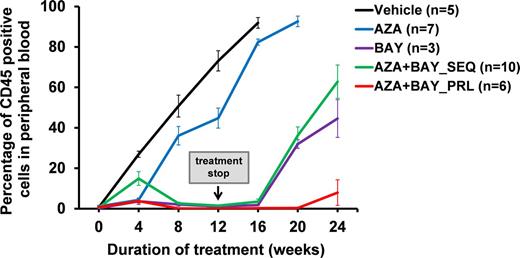
Results: Our IDH1 inhibitor reduced colony formation specifically in human IDH1 mutated AML cells, while IDH1 wildtype cells were not affected (IC50 100 nM). Combination of BAY-1436032 (100 nM) with azacitidine 100 nM further reduced colony formation by 50% in IDH1 mutated AML cells, while the IC50 for IDH1 wildtype AML cells was not reached even at 1000 nM azacitidine in the presence of 100 nM BAY-1436032. For in vivo experiments human AML cells from an IDH1 mutated AML patient were transplanted into sublethally irradiated NSG mice and treatment was started four weeks after transplantation. Leukemic cells in peripheral blood constantly increased in vehicle and azacitidine treated mice, while leukemic cells declined from week 4 until the end of treatment at week 12 in mice treated with BAY-1436032 alone as well as in the parallel and sequential combination groups treated with BAY-1436032 and azacitidine. However, in week 20 (8 weeks after stopping the BAY-1436032 treatment) leukemia relapsed in the BAY-1436032 alone as well as the sequential combination groups. Interestingly, the frequency of leukemic cells remained low in peripheral blood in mice treated in parallel with the combination of BAY-1436032 and azacitidine even at 24 weeks after starting the treatment (Figure 1). To obtain mechanistic insights into the efficacy of the combination treatment we treated human IDH1 mutant and IDH1 wildtype AML cells in vitro with either 100 nm BAY-1436032 or 100 nm azacitidine alone or in combination (in parallel). Vehicle treatment served as control. There were no significant differences in percentage of apoptotic cells between BAY-1436032 or azacitidine treatment as single agents or in combination. However, the proportion of cells in S phase of the cell cycle was synergistically decreased by the combination treatment compared with either monotherapy or vehicle. Gene expression profiling showed that the target genes of EGR1-3 and GFI1 transcription factors were upregulated by the combination treatment over BAY-1436032 treatment alone, while target genes of NFkB and its targets ELF1 and STAT1/3 were down regulated, suggesting synergy in induction of differentiation and inhibition of self-renewal of the combination treatment through distinct pathways.
Conclusion: Our study provides the first evidence of synergistic activity of an IDH1 inhibitor with hypomethylating agents through inhibition of the cell cycle by dysregulating EGR, GFI1 and NFkB signaling, and strongly argues for simultaneous application of the IDH1 inhibitor BAY-1436032 with azacitidine in future clinical trials. Clinical development is ongoing with phase 1 studies using BAY-1436032 in IDH1 mutant solid tumors and AML.
Figure 1: Development of human IDH1 mutated AML in peripheral blood of NSG mice treated with BAY-1436032 and azacitidine alone or in combination (PRL, in parallel; SEQ, sequential, i.e. first azacitidine days 1-5, then BAY-1436032 from day 6 onwards).
Chaturvedi: Bayer Pharma AG, Berlin, Germany: Research Funding. Kaulfuss: Bayer Pharma AG, Berlin, Germany: Employment. Panknin: Bayer Pharma AG, Berlin, Germany: Employment. Wagner: Bayer Pharma AG, Berlin, Germany: Employment, Equity Ownership. Jeffers: Bayer Pharma AG, Whippany, NJ, USA: Employment. Haegebarth: Bayer Pharma AG, Berlin, Germany: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal